Efficiency through synergy
As part of the lifecycle management of your brand, you have decided that a device optimisation exercise is required to bolster your brand’s value proposition and address a significant unmet need in the market. While checking in on the progress of this initiative with the various functional teams, you learn that the manufacturing team proposes the development of a new manufacturing process to reduce the cost of each unit, and ensure more devices can be produced and provided to a larger pool of patients worldwide. From the medical team perspective, the proposed approach is focused on redesigning the device to add extra features that address key patient needs and deliver an optimised experience. Although each team is developing effective plans for the task at hand, this situation highlights a common occurrence where various functional team processes may be conflicting rather than complementary.
This divergence between functional groups often arises because, without direction, a function will interpret an LCM goal through what it is able to do as opposed to what the brand or portfolio truly needs. A lack of alignment in LCM planning, one of the most cross-functional activities in our industry, is one of the biggest barriers to LCM success.
The graphic below demonstrates the range of functions that can and should be providing input into a successful LCM plan. And beyond this, further input will be required from regional and local teams, particularly as a product or portfolio moves to later stages of the lifecycle and greater degrees of geographic diversity must be managed.
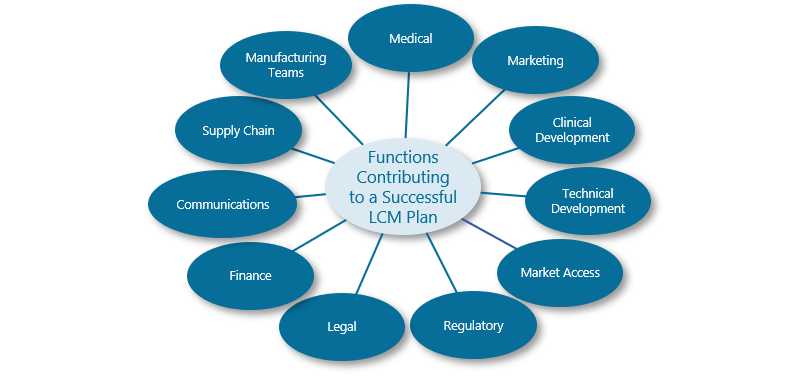
As soon as this range of functions, teams and individual personalities are involved in a process, alignment becomes the critical success factor. Ensuring this alignment in the earliest stages of LCM planning allows functional teams to work more efficiently and synergistically and maximises the chances of success.
An excellent example where the importance of working synergistically across functional teams is elevated even further, particularly from the outset, is within early stage indication prioritisation processes. Traditionally an exercise carried out by clinical development and medical teams, the inclusion of key commercial and access functions can provide important insight into the potential value and strategic fit of the indications under consideration, while regulatory and legal functions can support both the path to approval and potential for sustainable exclusivity. This early cross-functional input can pay dividends with streamlined clinical development that maximizes the long-term success of the product and portfolio. For more insight on planning ahead for multi-indication assets, including a tool to guide research and cross-functional decision-making, click here!
Once we have successfully enabled cross-team synergy, another integral component to consider is the need for support and buy-in from senior management.
LCM team structure
A key area in which this senior management support can be realised is through driving cross-functional commitment and alignment. This is done through the endorsement of and involvement in an LCM team structure that balances the need for broad involvement with the ability to execute a project within a realistic and reasonable timeline. In most cases the best structures involve three core layers:
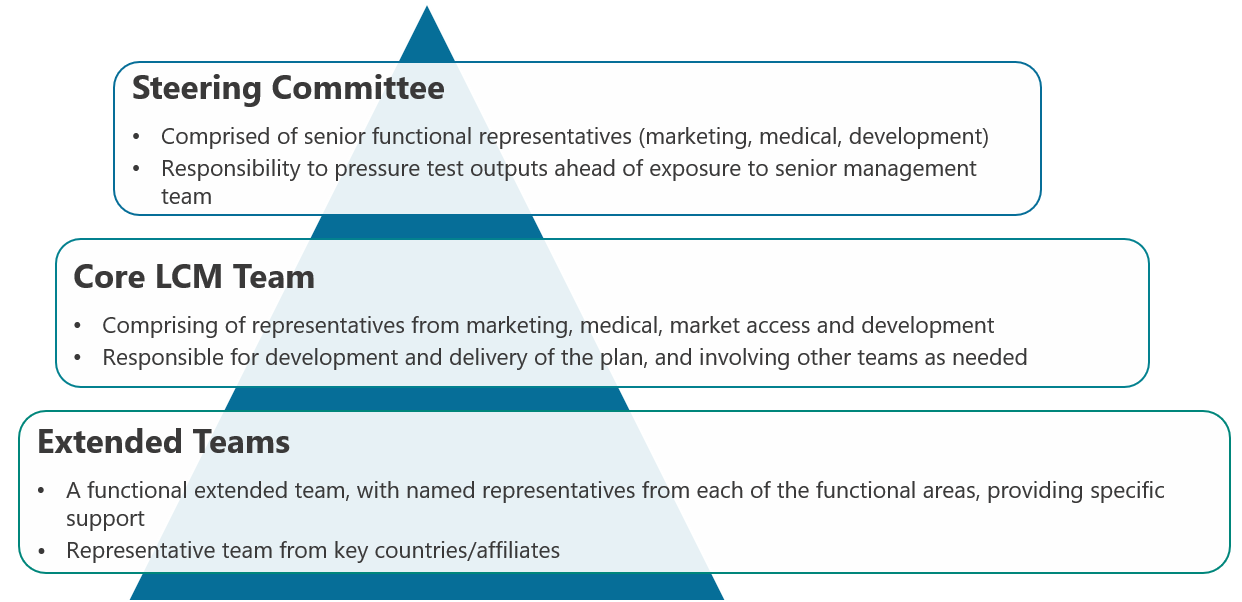
However, ensuring synergy between functions through the creation of an LCM organisation structure alone is not enough to guarantee the success of an LCM strategy. Alongside these structures, effective processes must also be established. How is this achieved to drive effective, rational, and aligned decision-making? Stay tuned for our next CSFs for LCM piece where we explore ‘Establishing an Effective Process for LCM’!